Brown Anole
Table of Contents
 |
| Norops sagrei male with its dewlap extended. Photo © Anole Annals |
1. Introduction
Norops sagrei, or the brown anole, as it is commonly known, is a small Neotropical lizard that has been recently introduced to Singapore in 2012 (Tan & Lim, 2012). It is native to Cuba and the Bahamas but has since been widely introduced and established around the world: Florida, Hawaii, Taiwan, Grand Cayman, Grenada, and Jamaica and recently, Singapore in 2012. Not all introduced populations come from their native source populations. In fact, brown anoles found in Grand Cayman, Grenada, Hawaii and Taiwan were brought in through secondary introductions from well-established populations in Florida (Kolbe et al., 2007). It is highly invasive in many countries but whether this exotic species will threaten local biodiversity in Singapore remains to be determined.
Note that it was only recently that the brown anole was reassigned to the genus Norops from Anolis by Nicholson et al. (2012). Prior to that, its scientific name was Anolis sagrei which is still commonly used to this day.
2. Description
The lizard has many distinguishing features, but only a selected few (the most prominent ones) are discussed here for ease of identification. Interested individuals can refer to the links provided at the end of the website for more useful readings. A table summarizing the distinctive physical attributes of the brown anole versus native lizards in Singapore is provided as a brief identification key in Section 3.
2.1 Coloration
The lizard’s scale color is controlled by melanophores. These are pigment-containing organelles found in fish, amphibians and reptiles. In particular, melanophores are responsible for the black or brown coloration of the brown anole. These lizards change colouration rapidly, often spanning their entire range of possible colors in only a few minutes, depending on the temperature, time of day, level of territorial aggression, or reproductive activity (Campbell, 2000). An example of how the anole changes coloration is that the lizard appears pale brown when warm, and darker brown to almost black when cool. This is because these ectothermic reptiles use darker colors to help absorb more heat from the sun when they are basking (Dailykos, 2013). The belly and throat are white and the edges of their eyelids are light beige (Norval et al., 2002).
Male color ranges from light gray to nearly jet-black, and plain-colored to covered dorsally with irregular dark patches or chevrons and a network of light lines. Mature females show a great change in colour as well, but usually possess a white dorsal stripe along the midline of their back. Along both sides of the line, black triangles fuse to form a diamond or zig-zag pattern that males lack (Campbell, 2000). Juveniles resemble females and it is difficult to distinguish between juvenile males and adult females. The purpose of the large range in variation of coloration is unknown, but it has been recently proven that females with an intermediate diamond-bar pattern dorsally had a higher survival rate (Calsbeek & Cox, 2010).
 |
| Juvenile with the white dorsal stripe and zig-zag pattern on both sides. Photo © Mark Yokoyama |
 |
| Highly varied coloration of the Norops sagrei. Red male with crests. Next to him is a female. Photo © Aaron Reedy |
 |
| Variation in the back patterns of Anolis sagrei in the Bahamas. From Calsbeek and Cox (2010). |
 |
| Juvenile with a network of white spots and lines along the dorsal region. Photo © Anole Annals |
2.2 Dewlap
 |
| Extended dewlap. Photo © Cyclical Core |
The most distinctive feature of the brown anole is its dewlap, a colorful flap of skin below the neck that is stretched like a drum over a highly modified hyoid apparatus and used for territorial and mating displays among males. Females generally have a functional dewlap, however, it is usually much smaller than that of males and usually not used. In the brown anole, the dewlap is reddish-orange with a white to yellowish border (Norval et al., 2002).
2.3 Snout-to-Vent Length
Snout-to-Vent length (SVL) is the distance from the tip of the rostrum to the anterior edge of the cloacal vent.
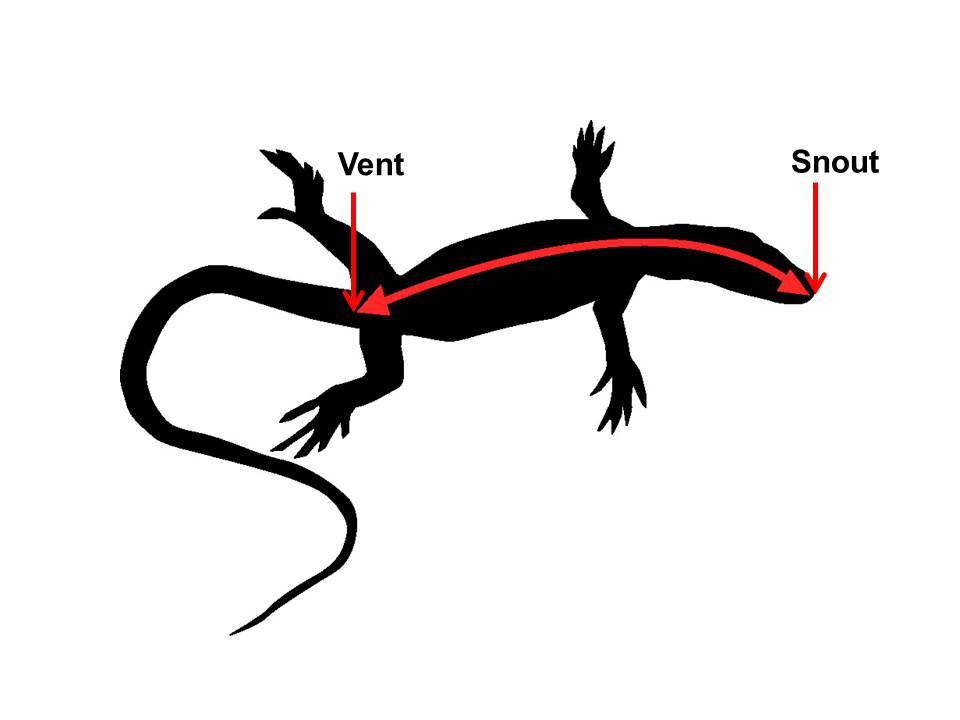 |
| Snout-to-Vent Length measurement. Picture © Laboratorie Informatique & Systema |
Upon maturity, males are approximately 64 mm in SVL and weigh 6 to 8 g, whereas females are about 48 mm SVL and weigh 3–4 g (Campbell, 2000).
2.4 Sexual dimorphism
This species is sexually dimorphic (Bartlett & Bartlett, 1997), with the males being larger, and generally darker with indistinct pale markings. Males also have a large dewlap or throat fan that varies from orange to red, and has a whitish or yellowish border. When the appendage is not erected, it appears as a reddish streak on the throat. Females and juveniles have either no dewlap or a poorly developed one. Some males have well-developed tail crests, erectile nuchal crests, and less distinct vertebral crests which females lack.
3. Diagnosis
Table 1. Summary of the differing physical attributes and ecology among the brown anole and other lizards in Singapore.
| Species |
Description |
Photos |
||||
| Brown Anole (Norops sagrei) |
SVL: Up to 6 cm Females and juveniles have one pale dorsal stripe. Males have a reddish-orange fan-like extensible dewlap. Commonly found on trunks and ground in urban areas. |
|
||||
| Changeable lizard (Calotes versicolor) |
SVL: 9.5cm Females have two pale dorsal stripes. Males adopt breeding colouration during mating season: black patches at the throat. Commonly found in low bushes and shrubs in urban areas. (Baker & Lim, 2008) |
 |
||||
| Draco sp. |
SVL: 9–10cm Males have a triangular erectile dewlap. Females have smaller ones. Males and females have patagia (a pair of wing-like flaps). Commonly found in primary forests of Bukit Timah and secondary forests of Central Catchment (Baker & Lim, 2008) |
|
||||
| Green crested lizard (Bronchocela cristatella), |
SVL: 13cm Bright green colouration and dark brown ear. Serrated dorsal ridge. Observed in Bukit Timah and Central Catchment Nature Reserve and other dense forests. (Baker & Lim, 2008) |
 |
4. Distribution
4.1 Globally
Distribution map of the brown anole. Note that the difference in colours of markings is due to the change in genus in 2012 from Anolis to Norops.
The native range of N. sagrei comprises the Bahamas, Belize, Cuba, Little Cayman, Cayman Brac, and Mexico (Schwartz & Henderson, 1991). introduced populations are thriving in the United States (Florida, Georgia, Louisiana, Tampa and Texas), and on the Caribbean (Grand Cayman, Grenada, and Jamaica) and Pacific (Hawaii and Taiwan) islands (Minton & Minton, 1984; Campbell, 1996; McKeown, 1996; Greene et al., 2002; Norval et al . 2002; Lever, 2003; Meshaka et al., 2004). Introduced N. sagrei populations are mainly from Cuba but some source populations originate from the Bahamas and Belize (Kolbe et al., 2004).
4.2 Singapore
In Singapore, the brown anoles can be found at Gardens by the Bay (1°17′4.97″ N, 103°51′53.86″ E) of Marina Bay South is a tourist attraction that is situated south of Singapore. The park spans 54 hectares and N. sagrei was first recorded there in October 2012 (Tan & Lim, 2012). It is surrounded by the Marina reservoir, a highway, golf course and commercial buildings. Construction of coastal land is ongoing to the east of the park (Tan & Lim, 2012). It houses over 25,000 species of plants. It is speculated that the lizard was spread to Singapore through the nursery trade (Kraus, 2009) which is one of the more common methods of introduction for this lizard.
Spot the brown anoles!
There are 14 anoles. Can you find them all? Answer |
| Anoles found in the Honduran Bay Island of Utilaa where it was introduced. Photo © Jonathan Losos |
5. Citizen science
Did you manage to spot all 14 anoles? Now you are good to go!
Play an active role in contributing to Singapore's biodiversity by helping us in monitoring the brown anole population! Report your sightings to iNaturalist and the information you share goes a long way to help researchers analyse and study the non-native population in Singapore.
6. Habitat
These habitat generalists are commonly found in urban open areas and perches low on trunks or artificial structures (Schoener, 1968; Losos, 2009). They prefer temperatures of around 29.2 to 32.5 °C (Lee, 1980).
6.1 Trunk-ground ecomorphs
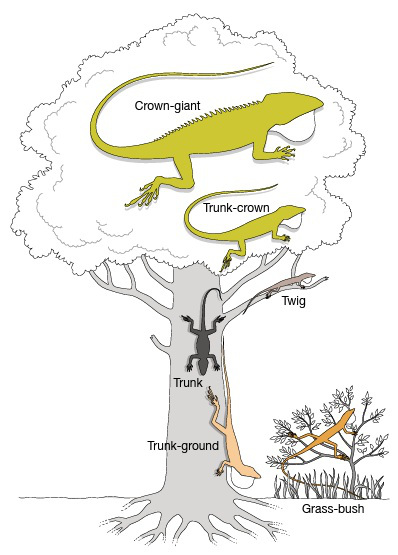 |
| Ecomorphs along the tree gradient. (Losos, 2009) |
According to William (1972), ecomorphs are species with the same structural habitat/niche, similar in morphology and behavior, but not necessarily close phyletically.
In the day, the brown anole adopts a head-down posture, and perches low on large trunks or fence-posts. It is a classic trunk-ground anole (Williams,1983). They are typically found up to heights of 6 feet (Losos, 2009).
 |
| Dewlap extended. In its classic head-down perch. Photo © Anole Annals |
7. Biology
7.1 Foraging mode
The brown anoles are sit-and-wait foragers surveying the ground from their perches low on tree trunks and rapidly dashing or jumping to the ground to apprehend prey that move within range. They tend to eat active prey (Losos, 2009).
Their classic survey posture is head down, forequarters lifted slightly off the surface, and hindlegs extended backwards. This position allows it to scan surroundings for prey, predators and conspecifics and to advertise their own presence to males or potential mates (Losos, 2009).
7.2 Diet
Their native diet consists mainly of small arthropods, annelids, and molluscs (Schoener, 1968; Schoener & Gorman, 1968).
Brown anoles consumed a wide variety of arthropods in Florida, including insects, amphipods, and isopods (Campbell, 2000). They also prey on other small vertebrates and hatchlings of green anoles (Campbell, 2000; Campbell & Gerber, 1996, Gerber & Echternacht, 2000). Likewise, many vertebrates consume brown anole adults (Campbell, 2000) and green anoles consume brown anole hatchlings (Campbell, 2000).
7.3 Mating and reproduction
Males perform a display in which they bob their heads and extend their dewlaps in a species-specific manner. The stereotypy of the head-bob cadence is important, as it appears to be a means for females to distinguish conspecifics from heterospecifics. Females respond by headbobbing or dewlapping (or both) and sometimes arch their neck to indicate receptivity. The male often bites the female on the neck and mounts on her back, swinging his tail around to the underside of the female’s tail and bringing their cloacae into close proximity. The male then everts one of his two intromittent organs, termed hemipenes and stored in the base of the tail, and inserts it into the female’s cloaca (Losos, 2009)
Reproduction of N. sagrei is largely unstudied, and most of their reproductive ecology is inferred from their relative the green anole, Anolis carolinensis (Lee et al., 1989).
7.4 Caudal autotomy
Caudal autotomy is a common defence mechanism amongst lizards, in which the animal loses its tail to escape from predators (Bateman & Fleming, 2009). This is facilitated by specializations in the vertebrae and attendant muscles and blood vessels that facilitate detachment with minimal trauma.
The brown anole can only regenerate its tail once, and this new section will contain cartilage rather than regenerating vertebrae of bone. However, the new tail is usually a dull grey and is smaller than the original tail (Campbell, 2000).
7.5 Display
 |
| Aggression between 2 brown anole males. Photo © Andrea Westmoreland |
 |
| Photo © Bird and Moon Comics |
The anole is visually-oriented and uses displays to communicate as they do not produce any audible sound. The anole uses headbobs, push-ups on top of dewlap extensions. These are usually used by the males for courtship, territorial display to other males. It is used as a “pursuit deterrent” signal to potential predators like snakes (Losos, 2009).
6. Reasons for invasiveness
6.1 Habitat generalist
They are habitat generalists but are known to prefer open habitats like suburbs or edge habitats (Campbell, 2002).
6.2 Females can store sperms
- Sperm production (male), mating (male & female) and ovulation (female) are often out of phase with one another in numerous reptile species. Hence, storage by either males or females promotes the wide dissemination, over time, of gametes produced in a narrow time window.
The ability for females to store sperm has been documented in Losos (2009). Calsbeek et al. (2007) observed captive Anolis sagrei laying eggs 107 days after they last mated. This allows the isolated anoles to colonise new areas.
6.3 Small numbers can establish large populations
The success of N. sagrei as an invader was demonstrated further by Losos and Spiller (1999) on Bahamian islands, where experimental founder populations of only five individuals flourished.
Twelve females and six males introduced to Degree-spoil Island, Florida, reached densities between 8,000 to 15,000 specimens ha-1 in only 4 years (Campbell & Echternacht, 2003). Today, approximately 50 populations with densities of approximately 12, 000 lizards per hectare are known from every county of peninsular Florida (Kolbe et al., 2004).
6.4 Dispersal across water
Schoener & Schoener (1984) tested 39 individuals in seawater tanks with waves to simulate choppy waters at sea and all brown anoles floated for an hour and 12 of them for 24 hours. Hence, their ability to disperse directly across water may be a reason for their notable success at invasion.
6.5 Reproduces at a fast rate
During breeding season, the female anole can lay clutches once every week (Losos, 2009).
7. Case Studies on Invasiveness
7.1 Taiwan
7.1.1 Reduced arthropod diversity
The invasion of the brown anole has severely affected the diversity and abundance of local terrestrial arthropods, such as orb spiders and arboreal insects. Lizard stomach content analysis showed that spiders comprised of 7% and insects 90% (more than 50% are ants) of the prey consumed
(Huang et al., 2008)
.
7.1.2 Altered ant community structure
Researchers tend to consider the interactions between vertebrate predators and ants to be weak. The present study examined the impact of the exotic invasive lizard, N. sagrei, on the ant community structure by manipulating the density of lizards within enclosures. The natural density of N. sagrei in the field was surveyed and used as the stocking density rate in the lizard-present sub-enclosures. Ant diversity in sub-enclosures with N. sagrei present was significantly different from that of enclosures where the lizards were absent, although the overall ant abundance did not differ significantly. The ant diversity difference was generated by a significant reduction of the ant species Pheidole fervens in sub-enclosures with N. sagrei present. Such an abundance change might be the result of direct predation by the lizards, or it might be generated by a foraging site shift by this ant. The results of this study thus demonstrated that the invasion of an exotic vertebrate can significantly alter the community structure (Huang et al., 2008).
7.2 Florida
7.2.1 Displacement of Anolis carolinensis
The presence of the brown anole has resulted in a decline in the native green anole, Anolis carolinensis, in Florida. feeding on the latter’s young which is intraguild predation (Gerber & Echternacht, 2000).
Predation experiments were conducted in cages, using freshly captured lizards, in which adult males of each species were presented with conspecific and heterospecific juveniles. Adult N. sagrei were significantly more likely to eat juveniles than were adult A. carolinensis (native in Florida) or A. conspersus (native in Grand Cayman), and significantly more likely to eat heterospecific than conspecific juveniles, whereas adult A. carolinensis and A. conspersus were not. Thus, the propensity for intraguild predation is asymmetrical in favor of introduced N. sagrei in Florida and Grand Cayman.
7.3 Grand Cayman
7.3.1 Competition-induced habitat shifts on A. conspersus
Comparisons with studies of habitat use prior to the arrival of N. sagrei indicate that in open habitats, where N. sagrei is now abundant, A. conspersus perches higher, but in closed habitats, where N. sagrei is absent, no change in perch height is evident (Losos et al., 1993).
7.4 What about Singapore?
The anole’s preference of disturbed and open areas should enable it to spread beyond the Marina Bay area to other urban areas of Singapore. Moreover, vehicular-rafting was the main mode of dispersal of the brown anole in Florida (Campbell, 1996), where it was previously introduced and might be a possible method in Singapore as well, as the Gardens by the Bay, being a tourist attraction, receives a high flow of traffic due to visitors.
However, it is also their habitat preference that should prevent the anoles from penetrating the secondary and primary forests at the Bukit Timah Nature Reserve and Central Catchment Nature Reserve where most of Singapore’s small native lizards, such as the earless agamid (Aphaniotis fusca) and the green crested lizard (Bronchocela cristatella), are found (Tan & Lim, 2012).
It is currently found in the same niche as another non-native lizard, the changeable lizard (Calotes versicolor). There might be possible interactions between the two lizards - be it intra-guild predation or competitors for food, but that would have to be better assessed with a long-term study.
Hence, it is not known whether the brown anole will turn invasive in Singapore, but past literature suggests that they do have the makings of an invasive species.
8. Taxonavigation
Kingdom: Animalia
Phylum: Chordata
Class: Reptilia
Order: Squamata
Family: Polychrotidae
Genus: Norops
Species: Norops sagrei (Duméril and Bibron 1837)
Synonyms: Anolis sagrei
8.1 Etymology
The generic name is from the Greek norops = brilliant or gleaming with reference to the bright color of the type species and is a translation of the Latin specific name of that species. (Wagler, 1830).
8.2 Type Information
No holotypes exists for this species. Holotypes are specimens used inside the original description of a new species. Since there are no holotypes, syntypes are used. These are the specimens listed inside the species description when holotypes are not available. Information is as follows:
MNHN2340 & MNHN6797 are kept at the National Museum of Natural History (France)
MCZ 2171 is at Museum of Comparative Zoology
9. Phylogenetic Tree
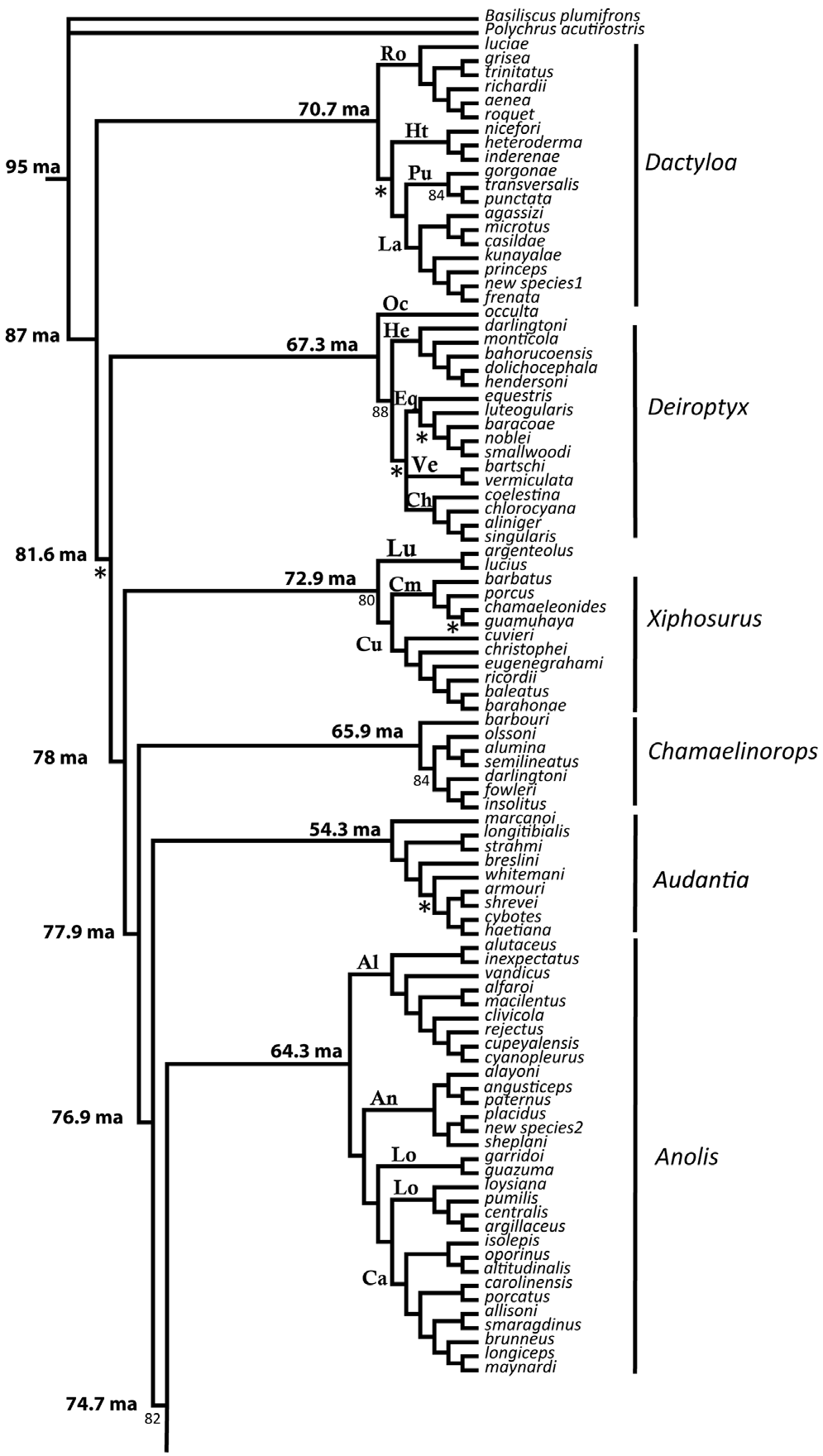 |
| Results of the parsimony analysis from combined morphological and molecular data (Nicholson et al., 2012). |
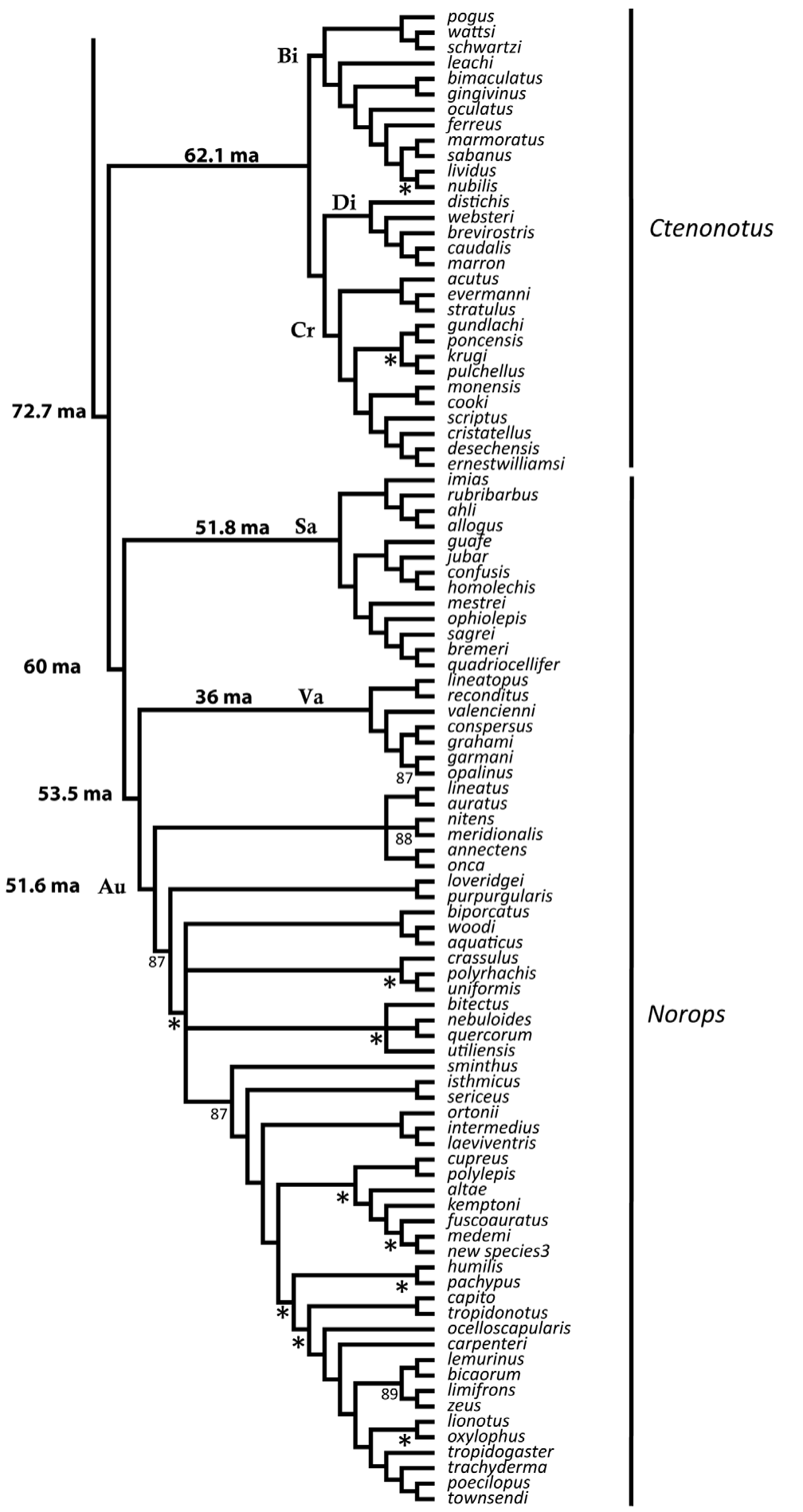 |
| Part 2. Results of the parsimony analysis from combined morphological and molecular data (Nicholson et al., 2012) |
The phylogenetic tree is reconstructed based on the results of a Bayesian phylogenetic analysis of molecular data by Nicholson et al (2012). Nodes with greater than 90% posterior probability have no symbol; posterior probabilities of 80–90% are indicated; less than 80% = *.
Morphological and molecular data from Poe (2004) and molecular data from Nicholson et al. (2005) were combined and reanalyzed under the Maximum Parsimony criterion. Nine outgroup OTUs were used in the combined data analyses: Anisolepis undulatus, Basiliscus plumifrons, Enyalius iheringii, Leiocephalus schreibersii, Polychrus acutirostris, P. marmoratus, Urostrophus melanochlorus, and U. vautieri. These data were analyzed with equal character weights. A heuristic search was conducted with Tree Bisection and Reconnection branchswapping. Because of the length of the dataset and number of included characters, the most parsimonious tree (mpt) search strategy was as follows: 1) used closest addition option to get starting random addition tree length and 2) 1000 repetition random addition searches with 1000 mpts saved. Only trees shorter than the starting tree length were saved, and this was repeated each time the tree length was reduced. The searches continued until five searches in a row failed to infer a shorter tree. Bootstrap proportions were estimated with 1000 replicates of the equally weighted data set. The combined morphological and molecular dataset consisted of 1580 characters for 240 taxa. Of these characters, 1198 were parsimony informative. The most parsimonious trees were 29,797 steps with a consistency index of 0.12 and a retention index of 0.48. Parsimony analysis of these data resulted in 4999 most parsimonious trees. The incongruent taxa are occulta, darlingtoni, and the sister taxa argenteolus + lucius. Many upper nodes are supported by bootstrap values of 80% or more, while deeper nodes possess bootstraps of less that 50% (nodes with greater than 80% bootstrap support show no value, between 50–80% the value is shown, and less than 50% = *). Apomorphies used to diagnose the clades were derived from one of the most parsimonious trees.
References
Baker, N. & K.K.P. Lim, 2008. Wild Animals of Singapore. A Photographic Guide to Mammals, Reptiles, Amphibians and Freshwater Fishes. Draco Publishing and Distribution Pte Ltd and Nature Society (Singapore). 180 pp.
Bartlett, R. D. & P. P. Bartlett, 1997. Anoles, Basilisks, and Water Dragons: A Complete Pet Owner’s Manual. Barron’s Educational Series, Inc., USA. 96 pp
Bateman, P.W., & P.A. Fleming, 2009. Invited review: To cut a long tail short: a review of lizard caudal autotomy studies over the last twenty years. Journal of Zoology, 277: 1-14.
Calsbeek, R., & R.M. Cox, 2010. Experimentally assessing the relative importance of predation and competition as agents of selection. Nature, 465: 613-116.
Campbell, T. S., 1996. Northern range extension of the Brown Anole (Anolis sagrei) in Florida and Georgia. Herpetological Review, 27:155-157.
Campbell, T. 2000. "The brown anole, Anolis sagrei" (On-line ). Institute for Biological Invasions Invader of the Month. Accessed November 28, 2013 at http://invasions.bio.utk.edu/invaders/sagrei.html.
Campbell, T. S. & A. C. Echternacht, 2003. Introduced species as moving targets: changes in body sizes of introduced lizards following experimental introductions and historical invasions. Biological Invasions 5:193-212.
Campbell, T.S., & G. P. Gerber, 1996. Anolis sagrei. Saurophagy. Herpetological Review, 27:200.
Gerber, G. P. & A. C. Echternacht, 2000. Evidence for asymmetrical intraguild predation between native and introduced Anolis lizards. Oecologia, 124:599-607.
Greene, B. T., D. T. Yorks, J. S. Parmerlee, Jr., R. Powell, & R. W. Henderson, 2002. Discovery of Anolis sagrei in Grenada with comments on its potential impact on native anoles. Caribbean Journal of Science, 38:270-272.
Huang, S.-C., G. Norval & I.-M. Tso, 2008. Predation by an exotic lizard, Anolis sagrei, alters the ant community structure in betelnut palm plantations in southern Taiwan. Ecological Entomology, 33:569–576.
Kolbe, J. J., Larson, A., & J. B. Losos, 2007. Differential admixture shapes morphological variation among invasive populations of the lizard Anolis sagrei. Molecular Ecology, 16:1571–1591.
Kraus, F., 2009. Alien Reptiles and Amphibians: A Scientific Compendium and Analysis. Springer Science and Business Media B. V. xii + 563 pp. Lever, C. 2003. Naturalized Reptiles and Amphibians of the World. Oxford University Press, New York, New York, USA.
Lee, J.C. 1980. Comparative thermal ecology of two lizards. Oecologia, 44:171–176.
Lee, J.C., Clayton, D., Eisenstein, S., & I. Perez, 1989. The reproductive cycle of Anolis sagrei in southern Florida. Copeia,1989: 930-937
Losos, J.B., and D. Spiller. 1999. Differential colonization success and asymmetrical interactions between two lizard species. Ecology 80: 252-258.
Losos, J. B., J. C. Marks, & T. W. Schoener, 1993. Habitat use and ecological interactions of an introduced and a native species of Anolis lizard on Grand Cayman, with a review of the outcomes of anole introductions. Oecologica, 95:525-532.
McKeown, S., 1996. A Field Guide to Reptiles and Amphibians in the Hawaiian Islands. Diamond Head Publishing, Inc., Los Osos, California, USA.
Meshaka, W.E., B.P. Butterfield, & J.B. Hauge, 2004. The Exotic Amphibians and Reptiles of Florida. Krieger Publishing Company, Malabar, Florida, USA.
Nicholson, K. E., 2005 . Historical biogeographic relationships within the tropical lizard genus Norops. In: Donnelly, M.A., Crother, B.I., Guyer, C., Wake, M.H. & White, M.E. (Eds.) Ecology and Evolution in the Tropics: A Herpetological Perspective. University of Chicago Press, Chicago, pp. 284–305.
Nicholson, K. E., Crother, B. I., Guyer, C. & Savage, J. M., 2012. It is time for a new classification of anoles (Squamata: Dactyloidae). Zootaxa, 3477: 1–108.
Norval, G., Mao, J.-J., Chu, H.-P., & L.-C. Chen, 2002: A new record of an introduced species, the brown anole (Anolis sagrei) (Duméril & Bibron, 1837), in Taiwan. Zoological Studies 41: 332–336.
Poe, S.,2004. Phylogeny of anoles. Herpetological Monographs, 18: 37–89.
Schwartz, A., and R. W. Henderson, 1991. Amphibians and Reptiles of the West Indies: Descriptions, Distributions,and Natural History. University of Florida Press, Gainesville, Florida, USA.
Schoener, A., & T. W. Schoener, 1984. Experiments on dispersal: Short-term floatation of insular anoles, with a review of similar abilities in other terrestrial animals. Oecologia 63(3): 289–294.
Schoener, T. W & G. C. Gorman, 1968. Some Niche Differences in Three Lesser Antillean Lizards of the Genus Anolis. Ecology, 49:819–830.
Schoener, T. W., 1968. The Anolis Lizards of Bimini: Resource Partitioning in a Complex Fauna. Ecology, 49:704–726.
Tan, H. H. & K. K. P. Lim, 2012. Recent introduction of the brown anole Norops sagrei (Reptilia: Squamata: Dactyloidae) to Singapore. Nature in Singapore, 5: 359–362.
Wagler, J.G.,1830. Natürliches system der Amphibien: mit vorangehender Classification der Säugethiere und Vögel: ein Beitrag zur vergleichenden Zoologie, J.G. Cotta, München, 354.






